Jesus Djalma PÉCORA
DDS., MSc., Ph.D., Full Professor at Ribeirão Preto Dental School,
University of São Paulo.
Manoel D. SOUSA-NETO
DDS, MSc., Ph.D., Head of the Clinics of the School of Dentistry, University
of Ribeirão Preto.
Danilo
M. Zanello GUERISOLI
DDS, Post-graduate student at Ribeirão Preto Dental School,
University of São Paulo, CNPq fellow.
Melissa
Andréia MARCHESAN
DDS, Post-graduate student at Ribeirão Preto Dental School,
University of São Paulo, CNPq fellow.
Department of Restorative Dentistry, Ribeirão Preto Dental School,
University of São Paulo
Av. do Café s/n
Ribeirão Preto, SP
Brazil
14040-904
Many authors have studied the effect of sodium hypochlorite on dentine permeability and concluded that this solution increases the permeability of dentine tissue1, 4, 12. The high surface tension of sodium hypochlorite solutions, similar to water, prevents an intimate contact between this liquid and dentine. A decrease of this surface tension has been studied by many authors7, 8, 10.
The purpose of this study was to evaluate the effect of different concentrations of sodium hypochlorite solutions on radicular dentine permeability, when associated with 0.1% lauryl diethyleneglycol ether sodium sulfate.
The samples were washed in running water for 24 hours in order to eliminate all thymol residues and then dried with air. The outer surface of the root was covered with 2 layers of cyanacrylate. Access to the pulp chamber was performed by routine clinical technique.
The teeth were randomly distributed into 9 groups of 5 teeth each and all teeth were instrumented as follows: determination of the working length in the apical limit, and use of 3 files in increasing sequential size from that which determined the anatomic diameter, with constant irrigation. The amount of irrigating solution used was 10.8 mL.
The teeth were then irrigated with 10.8 ml distilled water to remove any remaining solution used during instrumentation. The teeth were immersed in 10% copper sulfate for 30 min, in a vacuum for the first 5 min. The teeth were then dried with paper points and placed in a 1% rubeanic acid alcohol solution, for 30 min and in a vacuum for the first 5 min. Rubeanic acid reveals copper ions, forming a stained compound ranging in color from deep blue to black, depending on the quantity of copper ions present5.
Upon completion of this reaction to reveal the extension of penetration of copper ions, the tooth was placed in an acrylic resin block and 500-µm thick transverse sections were obtained with a diamond disk from the coronal, middle and apical regions. During the sectioning process, constant irrigation with water was used to prevent dentine burn.
According to established criteria, 2 samples of each region were used (the first and fourth slices of each radicular third). The slices were then filed under tap water to a thickness of approximately 100 µm and washed for 4 hours to eliminate dentine powder and other residues. After this, the samples were submitted to dehydration with increasing alcohol solution series, cleared in xylene and mounted on glass slides for microscopic examination.
The quantification of the penetration of copper ions was done by morphometry, with a 400-point grid (500 µm interpoint distance). Although the area had an irregular contour, it was considered a regular circle for evaluation purposes.
The number of points in the stained and non-stained areas of the dentine were counted, and the percent of copper ion penetration in the dentine was calculated by the following equation:
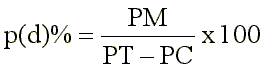
where: PM = points in the stained area, PT = total number of points counted, and PC = points in the canal area.
Preliminary tests were performed in order to verify if the sample distribution was parametric or not. Analysis of variance was performed using transformed data (square root). In order to detect the differences between the average values of the results, the data were submitted to the Tukey test.
Table 1: Average copper rubeanate penetration (%) in the dentine of
the studied teeth.
|
|
|||||||||
| Radicular thirds |
|
+ Surfactant |
|
+ Surfactant |
|
+ Surfactant |
|
+ Surfactant |
|
| Cervical |
|
|
|
|
|
|
|
|
|
| Middle |
|
|
|
|
|
|
|
|
|
| Apical |
|
|
|
|
|
|
|
|
|
Analysis of variance showed a statistically significant difference (p < 0.01) between the tested solutions and the three sectioning levels of radicular dentin (apical, middle and cervical).
The addition of 0.1% of lauryl diethyleneglycol ether sodium sulfate was sufficient to reduce the surface tension of the irrigating solutions by almost 50%.
The Tukey test revealed that the addition of the surfactant to the sodium hypochlorite solutions caused a significant increase in dentine permeability when compared to the same solutions without surfactant. The same test also showed that sodium hypochlorite solutions, pure or associated with surfactant, caused a significant increase of dentine permeability when compared to water.
The Tukey test indicated that the thirds were different and lower permeability values were obtained in the apical third.
Dentine permeability was increased significantly by the addition of surfactant to the sodium hypochlorite solutions (Tukey test). This test also demonstrated that sodium hypochlorite solutions, with or without surfactant, caused a significant increase of dentine permeability when compared to water.
These results can be explained as follows: a) water has a high surface tension (71.31 dynes/cm) and does not have any chemical action on the organic components present in the root canal; b) 0.5% sodium hypochlorite has a surface tension similar to water (68.8 dynes/cm), and at higher concentrations has a surface tension greater than water, but acts on organic components. The addition of 0.1% lauryl diethyleneglycol ether sodium sulfate reduces the surface tension of these solutions; c) The reduction of the surface tension increases the wetting capacity of the solutions, accelerating the chemical reaction8, 10.
The permeability of the thirds was statistically different and the apical third showed the lowest permeability values.