 (III)
|
In this study, the effect on the setting time of the addition of different grades of rosin and hydrogenated resin on the Grossman cement powder was evaluated. The experiments were carried following the American Dental Association specification number 57 for root canal sealers. For this analysis, different Grossman cement powders were prepared using different rosins (X, WW and WG) and hydrogenated resins (Staybelite and Staybelite ester 10). The study of the physicochemical properties of the Grossman cements obtained the different grades of rosins and hydrogenated resins interfere on the setting time of the cement. The hydrogenated resin, having a higher pH, increased the setting time of the cement when compared to the X, WW and WG rosins.
UNITERMS: Grossman cements, setting time
Powder: silver, hydrogenated resin and zinc oxide;
Liquid: eugenol and 4% zinc chloride solution.
In 1958, observing that the silver produced sulfides that darkened the
teeth, GROSSMAN eliminated it from the powder composition. Regarding the
liquid composition, he substituted the zinc chloride for almond oil. The
addition of vegetal oil had the purpose of retarding the setting time,
giving more time for the dentist to work on the root canal.
Still researching, GROSSMAN (1962) included anhydrous sodium tetraborate
to the powder of his cement, with the purpose to retard the setting time.
In 1974, this author concluded that the addition of almond oil to the eugenol
was not necessary, since the anhydrous sodium tetraborate was able to keep
a satisfying working time. Thus, alterations were made in the powder composition
and the liquid, and the final formula is shown below:
Powder:
Zinc oxide:
42%
Rosin or hydrogenated resin: 27%
Bismuth subcarbonate:
15%
Barium sulphate:
15%
Anhydrous sodium tetraborate: 1%
Liquid:
Eugenol
According to the requisites that a root canal sealing material must have, the properties and qualities can be divided into physicochemical, antimicrobian and biological.
The investigation of these properties became standardized since the publication of the specification number 57 of the American Dental Association (1983), avoiding the problems caused by the lack of standardization of the tests realized (BRANSTETTER & FRAUNHOFER, 1982), making the research results reproducible and also making possible accurate comparisons between the different materials and research results.
The different commercial brands of Grossman cements employ in their compositions hydrogenated resin or rosin, not having uniformity in the formula.
This way, it is necessary to study the influence of different grades of rosins and hydrogenated resins on the setting time of the Grossman cements, in order to inform which product shows the best physicochemical characteristics.
The cements tested were obtained from the formula proposed by GROSSMAN(1974), being the only difference between them the kind of rosin or hydrogenated resin (Table 1).
| Name | Manufacturer | Origin |
| Breutex - X | Eucatex | Brasil |
| Tipo WG | Madeitex | Brazil |
| Tipo WW | Coimbra | Portugal |
| Stabylite ester 10 | Hercules | USA |
| Stabylite resin | Hercules | USA |
The powder/liquid relations for the modified cements and spatulation
times were obtained as described by SOUSA NETO (1994) and are shown in
Table 2.
| TABLE 2: | Powder/liquid relations and spatulation times in seconds for the modified cements | ||||||||
|
|
|
|
|
|
|||||
| Staybelite ester 10 | 1,13 1,11 1,18 1,05 1,06 |
|
120 125 130 125 140 |
|
|||||
| Staybelite | 1,11 0,98 1,10 0,99 1,08 |
|
120 110 120 130 120 |
|
|||||
| Kind X | 0,92 0,94 0,96 0,93 0,98 |
|
120 110 110 120 120 |
|
|||||
| Kind WG | 0,90 0,92 0,89 0,94 0,85 |
|
120 120 140 130 120 |
|
|||||
| Kind WW | 0,80 0,75 0,78 0,82 0,82 |
|
140 120 135 125 115 |
|
|||||
In order to realize this test, rings of stainless steel with internal diameter of 10 mm and thickness of 2 mm were manufactured. The rings were fixed with wax in its external surface on a glass surface (1 x 25 x 75 mm).
After this the cement was manipulated, following the proportion expressed in Table 2 and placed inside the metallic ring, since it was completely filled.
After 120 ± 10 seconds from the beginning of the spatulation, the whole apparatus was placed over a grate inside a hermetically sealed plastic container. The device was kept at 37 ºC and 95% relative air humidity.
After 150 ± 10 seconds from the beginning of the spatulation, a 100 g, 2 mm active point Gillmore needle was laid vertically on the surface of the material.
This test was repeated each 60 seconds until the needle did not mark the surface of the cement. The setting time was considered as the amount of time passed from the beginning of the spatulation until the Gilmore needle did not leave any visible mark on the surface of the cement.
The setting time was considered as the average of 5 measures.
Results and discussion
The data obtained for the setting times, shown in Table 3, were submitted
to the statistical analysis. The sample parameter study pointed to a non-parametric
distribution. The Kruskal-Wallis test indicated a H0=0,03%
for a 1% significance.
TABLE 3. Flow test of the studied cements (data in millimeters)
|
|
(in seconds) |
|
||
| Staybelite ester 10 | 5520 5880 5160 5820 6180 |
|
||
| Staybelite | 3900 3540 4200 3300 3720 |
|
||
| Rosin X type | 2100 2160 2400 2040 2210 |
|
||
| Rosin WG type | 1060 1480 1360 1560 2240 |
|
||
| Rosin WW type | 1120 1280 1160 1220 1180 |
|
Once determined by the Kruskal-Wallis test that significant statistical
differences between the cements existed, the sample average comparison
was made, and the results are presented in Table 4.
| TABLE 4. | Setting time: Comparison between the sample average of the tested cements. | |||||
|
(two by two) |
|
|
||||
| Staybelite ester 10 X Staybelite |
|
|
||||
| Staybelite ester 10 X Rosin X kind |
|
|
||||
| Staybelite ester 10 X Rosin WG kind |
|
|
||||
| Staybelite ester 10 X Rosin WW kind |
|
|
||||
| Staybelite X Rosin X kind |
|
|
||||
| Staybelite X Rosin WG kind |
|
|
||||
| Staybelite X Rosin WW kind |
|
|
||||
| Rosin X kind X Rosin WG kind |
|
|
||||
| Rosin X kind X Rosin WW kind |
|
|
||||
| Rosin WG kind X Rosin WW kind |
|
|
||||
| *Significant to the level of 0,01 = 4.6068 ns= non- significant | ||||||
The setting times of the cements obtained from hydrogenated resins and different grades of rosin are statistically different between them.
SOUSA NETO (1997) analyzed the pH and conductivity
of different grades of rosins and hydrogenated resins to verify their relation
to the different physicochemical properties, and these data are shown on
Table 5.
| TABELA 5. | pH and conductivity values of the different grades of rosin and hydrogenated resins versus time | |||||||||||||||||
|
|
|
|
||||||||||||||||
| 1 | 2 | 5 | 10 | 20 | 30 | 60 | 1 | 2 | 5 | 10 | 20 | 30 | 60 | |||||
| Stabylite Éster 10 | 3.9 | 4.5 | 5.0 | 5.3 | 5.6 | 5.6 | 5.6 | 14 | 16 | 17 | 29 | 59 | 62 | 68 | ||||
| Stabylite | 4.7 | 4.9 | 4.9 | 5.1 | 5.1 | 5.1 | 5.1 | 24 | 25 | 34 | 44 | 44 | 47 | 59 | ||||
| Rosin X type | 5.2.. | 5.2 | 5.2 | 5.2 | 5.1 | 5.2 | 5.0 | 26 | 29 | 39 | 45 | 65 | 88 | 105 | ||||
| Rosin WG type | 4.2 | 4.7 | 4.7 | 4.8 | 4.8 | 4.7 | 4.7 | 66 | 145 | 270 | 336 | 415 | 580 | 710 | ||||
| Rosin WW type | 4.6 | 4.3 | 4.2 | 4.3 | 4.0 | 4.0 | 3.6 | 32 | 98 | 141 | 167 | 193 | 223 | 347 | ||||
Conductivity is a property that indicates the amount of ions present in a solution. The higher the value, higher the amount of ions in the medium.
When different grades of rosins were compared, we observed that the X kind presented low conductivity. This characteristic can be justified by the purification method that this rosin is submitted during its manufacturing process.
The rosin X kind has an uniform light yellow color, different from the grades WG and WW, which have a dark yellow color with differences in pigmentation, indicating the presence of impurities (inorganic ions)
The WG rosin has a high conductivity value, indicating high quantities of inorganic ions in its composition.
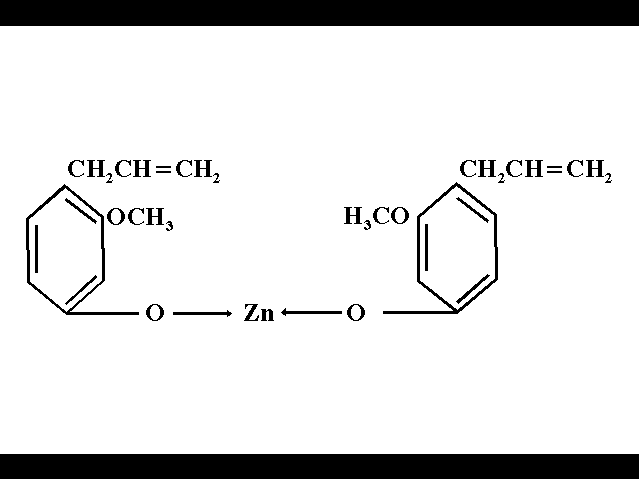
The hydrogenated resins are obtained from the rosin hydrogenation. This process consists of adding hydrogen to a molecule, by the reaction with gaseous hydrogen, with or without the presence of a catalyst, lowering the number of double links of an unsaturated chain.
The low conductivity found in the hydrogenated resins is due to the hydrogenation process, which removes the impurities from the rosin and makes the chain saturated. These resins showed the least quantity of inorganic ions among the studied rosins.
The GROSSMAN cement (1958, 1962 and 1974) is a zinc-oxide eugenol based cement. The other components are added in order to obtain better physicochemical properties. Thus, the setting reaction is due to the reaction between the zinc and the eugenol.
The setting reaction of the zinc and eugenol is basically an ionic reaction, where the eugenol acts as a proton donator (H+). The phenolic hydrogen in eugenol is substituted by the zinc ions to form a zinc-oxide eugenol chelate (FRAGOLA et al, 1979).
The setting mechanism of the zinc-oxide eugenol based cements is the result of equimolars mixtures of zinc oxide and eugenol, consisting of zinc-oxide involved in a long crystal matrix of zinc eugenolate chelate. Any excess of eugenol is absorbed either by eugenolate and zinc oxide (BRAUER et al, 1967)
SAVIOLI (1992) clearly showed the effect of rosin on the setting reaction of the zinc oxide based cement, reinforcing GROSSMAN findings. The addition of rosin to the powder acts as an accelerator.
Based on the works of FRAGOLA et al (1979), BRAUER et al (1967), GROSSMAN (1982) and SAVIOLI (1992) the influence of the pH on the setting reaction can be explained by this manner:
ZnO + H2O
®
Zn (OH)2 (I)
Zn (OH)2
+ 2H+ ®
Zn 2+ + 2H2O
(II)
 (III)
|
The reaction between zinc and eugenol is of an ionic nature.
The pH indicates a higher hydrogenionic concentration (H+). Thus, the higher the quantity of H+, the more quick the reaction (II) that produces zinc in its ionic form (Zn2+).
A higher quantity of Zn2+ makes the reaction (III) quicker, accelerating the setting time. This mechanism explains the absence of hardening in cements that contain hydrogenated resins with the pH around 7 (neutral). In this pH there is no H+ excess in order to release Zn2+. GROSSMAN (1982) relates that the cements containing hydrogenated resins with pH around 7 in their formulas did not set.
In this experiment, the long time the cements prepared with hydrogenated resin took to set can be explained by its pH and low conductivity. The pH of the hydrogenated resins is higher than the rosins and their conductivity is lower.
The rosins, due to the concentration of abietic acid, have low pH — indicating a high concentration of H+ in the medium., accelerating the reaction between zinc oxide and eugenol.
BATCHELOR & WILSON (1969) stated that consistency and setting time in the cements are related and both are affected by variables like temperature and humidity. Thus, before the realization of the tests in this study, the powder-liquid ratio was established to achieve the ideal consistency for the cements as stated by GROSSMAN (1974).
The setting of the material on the glass surface is not related to the clinical conditions, where the temperature and oral humidity will interfere in the process. The amounts of material used in the clinical and laboratory conditions are also different. In order to avoid variations in the results, ADA specifications were used for ambient conditions: 37 ºC and 95% relative humidity.
In this study, it was observed that different grades of hydrogenated
resins and rosins interfere on the setting time of the cements. The hydrogenated
resin has a higher pH than the rosin, increasing the setting time when
compared to the rosins kind X, WW and WG, more acid.
1. Different grades of hydrogenated resin and rosin interfere on
setting time
1.2. The setting time follows the crescent order: WW rosin, WG rosin, X rosin, Staybelite ester 10 resin and Staybelite resin.
No presente estudo, analisou-se o efeito da adição de
diferentes tipos de breus e resinas hidrogenadas ao pó do cimento
de GROSSMAN sobre o tempo de endurecimento. Os experimentos foram realizados
de acordo com a Especificação 57 para materiais obturadores
de canais radiculares da American Dental Association (ADA). Para análise,
foram aviados pós do cimento de GROSSMAN com diferentes tipos de
breu (X, WW e WG) e resinas hidrogenadas (Stabylite e Stabylite éster
10). Os estudos das propriedades físico-químicas dos cimentos
tipo GROSSMAN obtidos de diferentes tipos de breus e resinas hidrogenadas
interferem no tempo de endurecimento do cimento. A resina hidrogenada,
obtida do processo de hidrogenação tem o pH mais alto, provocando
um aumento do tempo de endurecimento do cimento em relação
aos breus tipo X, WW e WG, que têm pH mais ácido.
UNITERMOS: Cimento de Grossman, Tempo de Endurecimento
2. BATCHELOR, R.F. & WILSON, A.D. Zinc oxide-eugenol cements. I. The effect of atmosferic conditions on rheological properties. J. Dent. Res., v.48, n.5, p.883-7, 1969.
3. BRANSTETTER J. & FRAUNHOFER, J.A. The physical properties and sealing action of endodontic sealer cements: a review of the literature. J. Endod. 8: 312-316, 1982.
4. BRAUER, G.M. New developments in zinc oxide-eugenol cement. Annals of Dentistry, v.26, n.2, p. 44-50, 1967.
5. FRAGOLA, A.; PASCAL, S.; ROSENGARTEN, M.; SMITH, A.; BLECHMAN, H. The effect of varying particle size of the components of Grossman's cement. J. Endod., v. 5, n. 11, p. 336-9, 1979
6. GROSSMAN, L. I. An improved root canal cement. J. Amer. Dent. Assoc., v. 56, n. 3, p. 381-5, 1958.
7. GROSSMAN, L. I. Endodontic Practice. 8 ed. Philadelphia, Lea & Febiger, p. 299-300, 1974.
8. GROSSMAN, L. I. Filling root canals with silver points. D. Cosmos, v. 78, n. 7, p. 679-87, 1936.
9. GROSSMAN, L. I. Root canal therapy. 2 ed. Philadelphia, Lea & Febiger, p. 281, 1946.
10. GROSSMAN, L. I. Setting time of selected essential oils with a standard root canal cement powder. J. Endod., v. 8, n. 6, p. 277-9, 1982.
11. GROSSMAN, L. I. The effect of pH of rosin on setting time of root canal cements. J. Endod., v. 8, n. 7, p. 326-7, 1982.
12. GROSSMAN, L. I. Algunas observaciones sobre obturación de conductos radiculares. Rev. Asoc. Odont. Argent., v. 50, n. 2, p. 61-6, 1962.
13. GROSSMAN, L.I. Physical properties of root canal cements. J. Endod., v. 2, n. 6, p. 166-75, 1976.
14. MOLNAR, E.J. & SKINNER, E.W. A study of zinc oxide-rosin cements. I. Some variables which affect the hardening time. J. Amer. Dent. Assoc., v. 29, n. 5, p. 744-51, 1942
15. SAVIOLI, R. N. Estudo da influência de cada componente químico do cimento de Grossman sobre as suas propriedades físicas. Ribeirão Preto, 1992. 123 p. Tese (Mestrado). Faculdade de Odontologia de Ribeirão Preto da Universidade de São Paulo.
16. SOUSA NETO, M. D. Estudo da influência de diferentes tipos de breus e resinas hidrogenadas sobre as propriedades físico-químicas do cimento obturador dos canais radiculares do tipo Grossman. Ribeirão Preto, 1997. 108 p. Tese (Doutorado). Faculdade de Odontologia de Ribeirão Preto da Universidade de São Paulo.